Research Interest - ER stress response-
We identified OASIS as a novel ER stress transducer. OASIS is a basic leucine zipper (bZIP) transcription factor of the CREB/ATF family with a transmembrane domain that allows it to associate with the ER. The molecule is cleaved at the membrane in response to ER stress, and its cleaved amino-terminal cytoplasmic domain, which contains the bZIP domain, translocates into the nucleus where it activates the transcription of target genes that are mediated by ER stress-responsive and cyclic AMP-responsive elements. Intriguingly, OASIS was induced at the transcriptional level during ER stress in astrocytes of the central nervous system, but not in other cell types examined. Furthermore, overexpression of OASIS resulted in induction of BiP and suppression of ER-stress-induced cell death, whereas knockdown partially reduced BiP levels and led to ER stress in susceptible astrocytes. Our results reveal pivotal roles for OASIS in modulating the unfolded protein response in astrocytes, and the possibility that cell type-specific UPR signalling also exists in other cells (Fig. 2).
We are currently generating OASIS knockout mice to elucidate the physiological functions of OASIS in vivo.
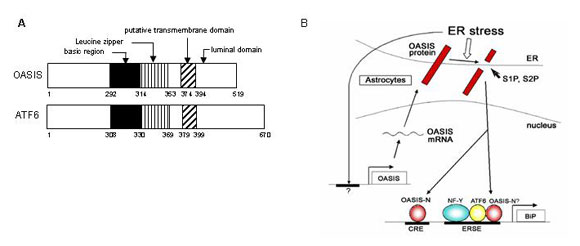
Fig. 2 The comparison of the structure between OASIS and ATF6, and Regulation of unfolded protein response by OASIS in astrocytes.
Kondo, et al. Nature Cell Biology 7: 186-94, 2005.
Murakami, et al. Journal of Neurochemistry 96: 1090-1100, 2006.
Saito, et al. Antioxidants & Redox Signalimg 9: 563-571, 2007.
A new group of ER stress transducers including CREBH, AIbZIP/Tisp40 and Luman, has been identified whose members are homologous to OASIS (Fig.3). These transducers are processed in a manner similar to that of OASIS and ATF6, which in response to ER stress are sequestered in cellular membranes and activated by RIP. Recently, we also identified BBF2H7, an ER-resident transmembrane protein with the bZIP domain in the cytoplasmic portion and structurally homologous to OASIS. Interestingly, although BBF2H7 protein is not expressed in normal conditions, it is markedly induced at the translational level during ER stress, suggesting that BBF2H7 might contribute to only the late phase of UPR signaling. In a mouse model of focal brain ischemia, BBF2H7 protein is prominently induced in neurons in the peri-infarction region. Furthermore, in a neuroblastoma cell line, BBF2H7 overexpression suppresses ER stress-induced cell death, while siRNA knockdown of BBF2H7 promotes ER stress-induced cell death. Taken together, BBF2H7 could play important roles in preventing accumulation of unfolded proteins in damaged neurons.
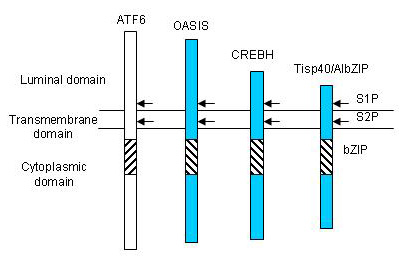
Fig.3 The structures of OASIS family members.
Kondo, et al. Molecular and Cellular Biology 27: 1716-1729, 2007.
