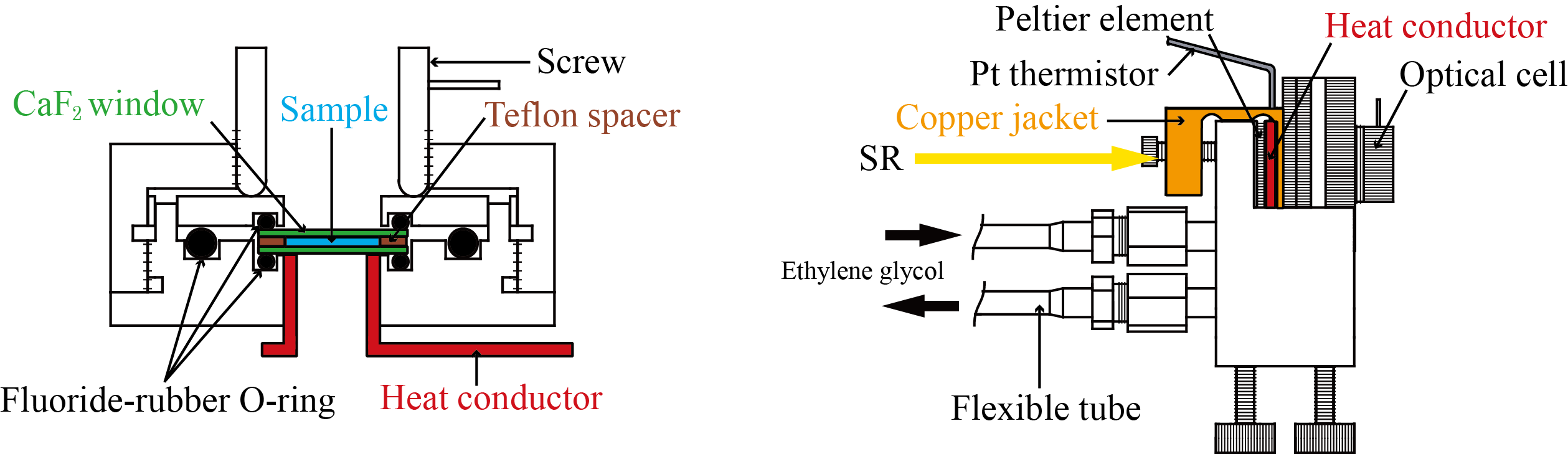
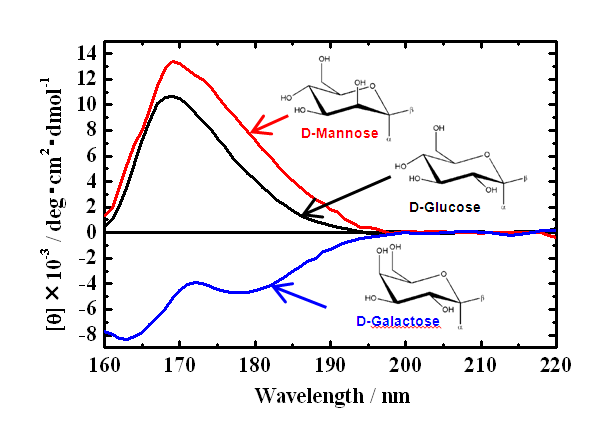
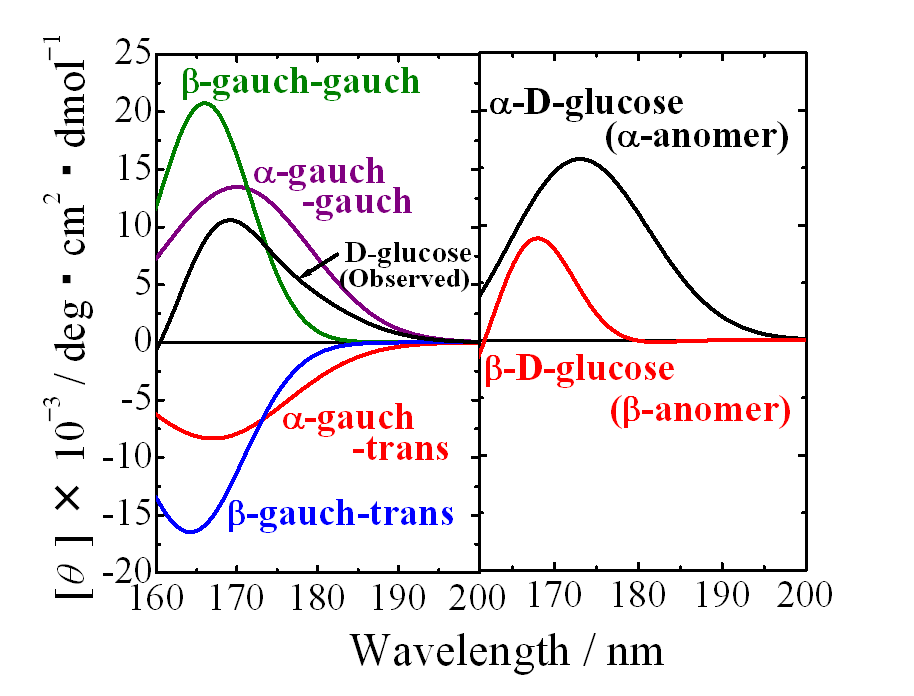
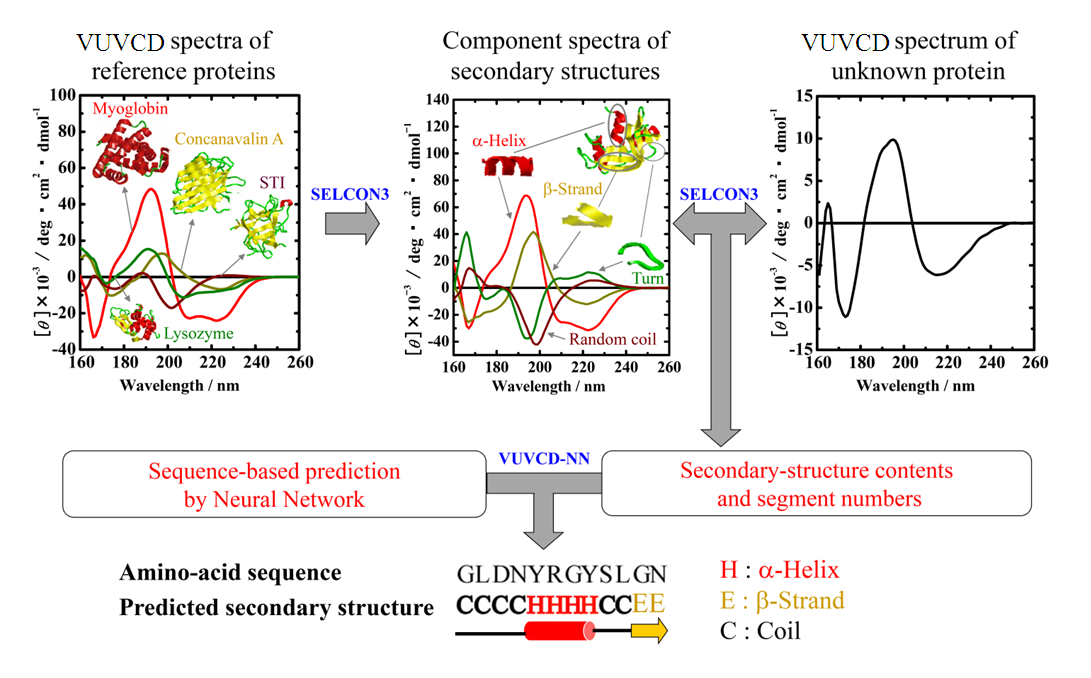
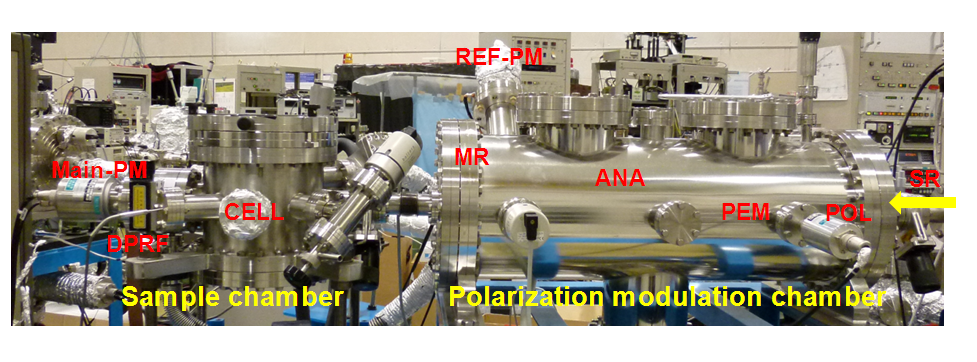
Optical device
SR-VUVCD spectrophotometer consists of two separate vacuum chambers (at10-4 Pa; a polarization modulation chamber and a sample chamber). All optical devices of the spectrophotometer are set up under a high vacuum (10-6 Torr), in order to avoid the light absorption in the VUV region by air and water vapor. The SR light is separated into two orthogonal linearly polarized light beams by a linear polarizer (POL, MgF2). Both linearly polarized light beams are modulated into circularly polarized light at 50 kHz by a photo-elastic modulator (PEM, LiF). The main light beam is led to the sample cell and the CD signal is detected by a photomultiplier tube (Main-PM), which is covered by an MgF2 window. The CD signal is rectified and amplified by the lock-in amplifier (LIA), and finally recorded on a personal computer. Another light beam from the PEM is detected as a reference signal by another photomultiplier tube (Ref-PM) after being modulated into a linearly polarization light beam at 100 kHz by a linear polarizer (ANA, MgF2), to obtain optimal driving voltages for the PEM. Further, this reference signal is used to enhance the stabilization of the LIA through the synchronization and rectification of the CD signal detected by the Main-PM.
 |
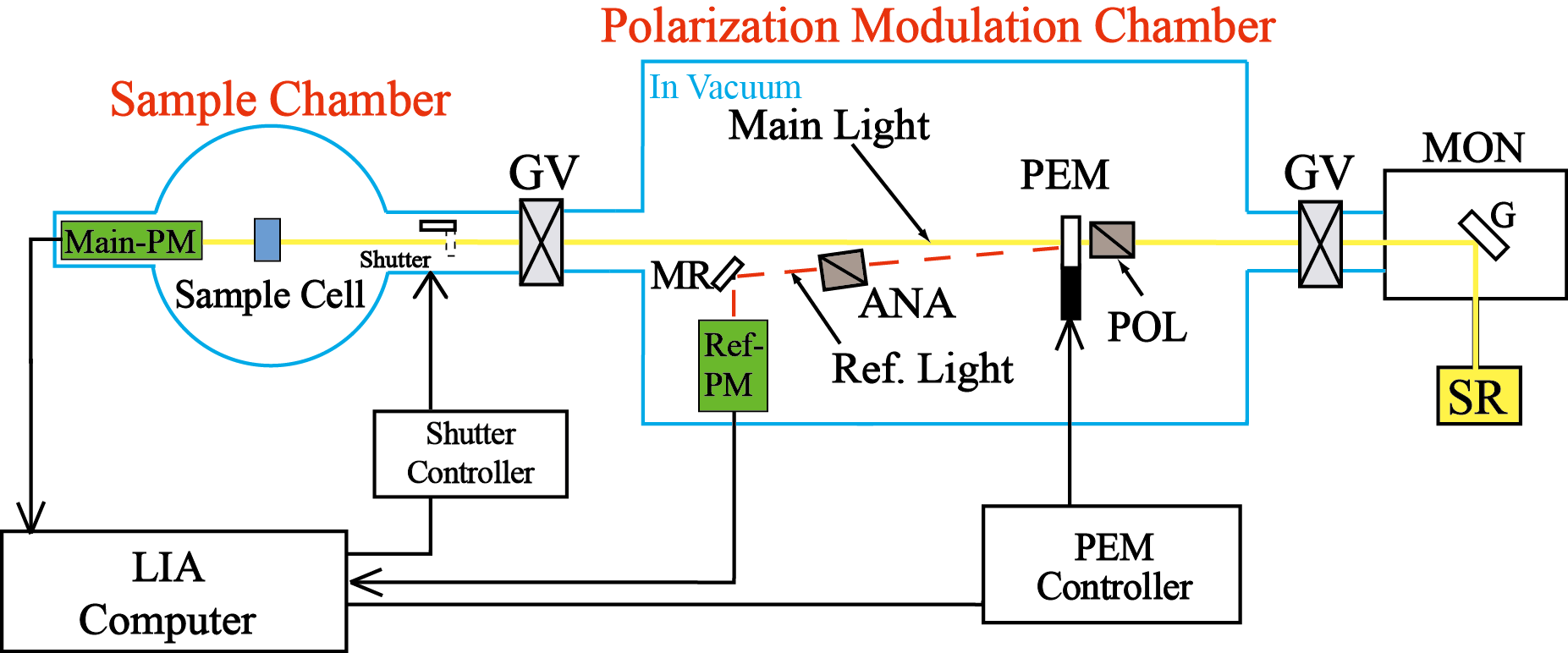 |
ANA, analyzer; G, grating; GV, gate valve; LIA, lock-in amplifier; MON, monochromator; MR, mirror; PEM, photoelastic modulator; PM, photomultiplier tube; POL, polarizer; S, shutter; SR, synchrotron radiation.