Metal-nonmetal transition in supercritical fluids
|
Å@As is well known, the boiling point gradually rises when pressure is applied, but above a certain pressure, the first-order phase transition from liquid to gas no longer occurs, and the liquid and the gas phases are not distingushable This indistinguishable state called supercritical fluid appears above the critical temperature and pressure (this boundary point is called the critical point). This means that if the temperature and pressure are well controlled, it is possible to reduce the density continuously from the liquid near the melting point to the dilute gas by more than three orders of magnitude reduced by circumventing the critical point. A liquid never expands uniformly at the atomic level, but if we consider it as an average, the distance between atoms and molecules can be expanded ten times or more in the process of this volume expansion. Å@It is very important from the standpoint of condensed matter physics to investigate what kind of changes occur in the structure and electronic states of fluids when such a large volume expansion occurs. In particular, in the case of semiconductoring or metallic fluids, the physical properties change greatly as the volume expands. In fact, the fluid Se (selenium), which behaves as a semiconductor just above the melting point, undergoes a semiconductor-metal-insulator transition in the supercritical region. Alkali metal fluids such as Rb (rubidium) and Cs (cesium) and a divalent metal Hg (mercury) undergo a metal-nonmetal transition near the critical point. More interestingly, these fluids also show very peculiar behaviors in their thermodynamic properties, for example, the shape of the gas-liquid coexistence curve plotted on the density-temperature plane is greatly distorted, and the correspondence seen in ordinary fluids is not indicated. Interesting facts such as the law of states and the law of linear diameters do not hold in the supercirtical metallic fluids, and the abnormal behavior of the critical exponent are observed. In this way, semiconductoring and metallic fluids have interesting characteristics different from ordinary fluids, and further development of future research is highly expected. |
 |
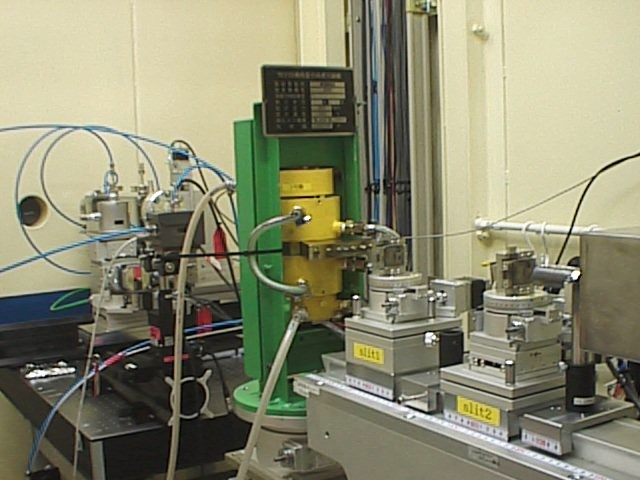 |
| SPring-8 | Apparatus for x-ray diffraction (SPring-8Åj |
 |
 |
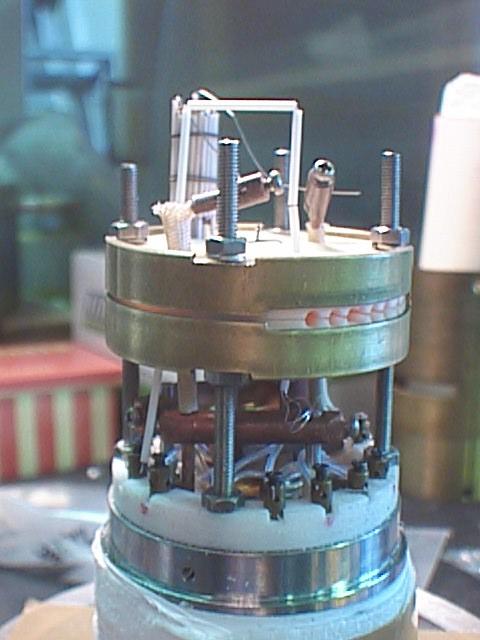
| Inside of a high-pressure vessel | sapphire cell |
Liquid-Liquid phase transition
|
High-density and low-density phases have been found in amorphous ice under low-temperature and high-pressure [1], and supercooled water under low-temperature and high-pressure is expected to undergo a first-order phase transition from a low-density phase to a high-density phase. The first-order phase transition is being confirmed by computer simulations and experiments with microwater that has expanded the supercooling temperature range. A first-order phase transition was also found in the high-pressure phase of liquid phosphorus [2]. more in Japanese. [1]O. Mishima, L. D. Calvert, and E. Whalley, Nature 314, 76 (1985) DOI [2]Yoshinori Katayama, Takeshi Mizutani, Wataru Utsumi, Osamu Shimomura, Masaaki Yamakata & Ken-ichi Funakoshi Nature 403, 170(2000) DOI |