Overview
We are providing new strategies for polymer synthesis based on organometallic chemistry. Especially, we are focusing on the development of scaffolds that can be precursors of counteranion in coordination polymerization catalyst system. A group member can learn various techniques and methods such as standard Schlenk techniques, NMR spectroscopy, X-ray crystallography, and computational chemistry. The three main projects shown below are currently ongoing.
Fluorinated organoborane catalyst
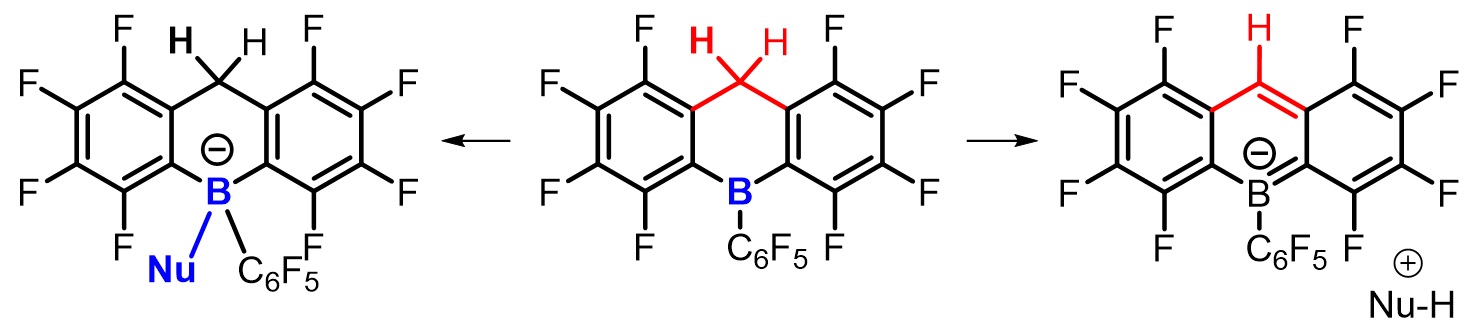
Fluoroarylboranes are one of the most important classes of Lewis acids, which is used for a variety of catalytic reactions including coordination polymerization, cationic polymerization, and ring-opening polymerization. Fluoroarylborane involves in various catalytic mechanisms and is often combined with transition-metal catalysts or bulky Lewis bases. Recently, we developed a new fluoroarylborane compound, fluoroboradihydroanthracene, which shows conventional Lewis acidity as well as strong Bronsted acidity, although the compound is neutral and highly soluble in low dielectric constant solvents. This compound thus possesses high activation property of coordination polymerization catalysts and enabled the synthesis of ultrahigh molecular weight polypropylene (Mn > 10^6). Currently, some projects about the application of this compound as a catalyst accessing environmental-friendly polymeric materials are ongoing.
Development of recyclable hydrocarbon polymers using dynamic covalent bonds
Boronic acid functionalities in the synthetic polymer have recently been paid great attention because they are applicable to reversible star assembly and crosslinking using their reversible transformation abilities. We recently have achieved the incorporation of boronic acids into the chain end or main chain by the direct copolymerization of vinyl monomers bearing boronic acid moieties. Especially, random copolymerization of boronic acid-containing comonomers has been challenging because boronic acid is not tolerant to the coordination polymerization conditions, and our comonomer design enabled to copolymerize boronic amide comonomer and propylene in highly isospecific manner. These copolymerization strategies were currently applied for reprocessable hydrocarbon rubber materials like ethylene-propylene rubber.
An inteview regarding this topic is on the University webpage.
Chemistry of Methylaluminoxane
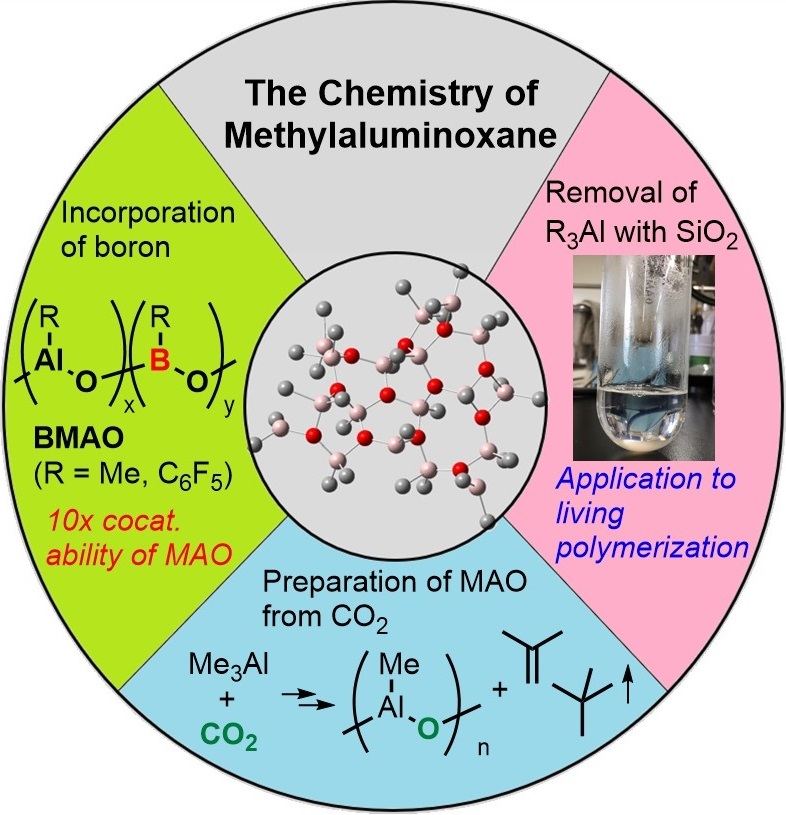
Methylaluminoxane (MAO) is a very important Lewis acid for coordination polymerization because it shows versatile activation properties toward transition metal-based catalysts in coordination polymerization chemistry. Along with recent emerging understandings of the structure and the origin of activation properties, we are developing some new preparation or modification methods of MAO which improves cocatalyst performance and/or easiness to handle, such as 1) incorporation of boron into the main chain of MAO, 2) practical removal method of trialkylaluminums from MAO, and 3) preparation of MAO from CO2.
Lanthanide complex catalysts for diene polymerizations
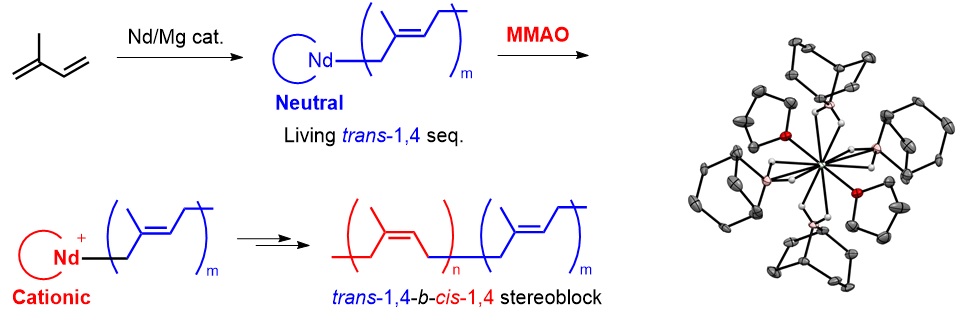
Precise control of the primary structure of polyisoprene and polybutadiene is important because their thermal, physical, and mechanical properties are greatly influenced by their stereoregularity. With a series of new organoborohydrido neodymium complexes, we have opened up the way to synthesize new poly(conjugated diene)s which bears tailored microstructure, molecular weight, and its distribution. For example, by controlling the valence of neodymium borohydride catalyst during polymerization with methylaluminoxane, we succeeded in synthesizing steredioblock polyisoprene and polybutadiene which is consist of cis-1,4 and trans-1,4 specific blocks.